How To Get A Prescription For Mounjaro

The Mounjaro Experience, Designed For Patients Like Juliaane
Come across JULIA
Unhappy with her A1C and weight, concerned about life with Blazon two Diabetes (T2D)*

Actor portrayal of an adult woman with blazon two diabetes
Adult woman with type 2 diabetes continuing with her arms crossed
*A patient you may run into.
- Recently diagnosed with T2D
- Non to her A1C goal on metformin
- With her T2D, she struggles with her weight despite her efforts with nutrition and exercise
An elevated A1C and excess weight contribute to the burden of T2Dtwo-four
Become patients started on Mounjaro

Offset your patients with a sample (two.5 mg) and a prescription (v mg)
Provide patients a 1-calendar month† sample of Mounjaro ii.5 mg
Samples include 4 once-weekly pens and information about the Mounjaro Savings Program.
See Savings Card Terms and Weather condition.
†One month is defined every bit 28 days and 4 pens.
Starting and continuing once-weekly Mounjaro‡
The 2.v mg dose is for treatment initiation and is not intended for glycemic command.

Prototype depicting escalation of Mounjaro. Starting dose is 2.5 mg once weekly for 4 weeks. Continue to 5 mg one time weekly for at least 4 weeks. If additional glycemic control is needed, dose tin be increased to 7.5 mg one time weekly for at least 4 weeks, then ten mg once weekly for at least iv weeks, and then 12.five mg one time weekly for at least 4 weeks, and so xv mg once weekly as a maximum dose.
The ii.5 mg dose is for treatment initiation and is non intended for glycemic command.
‡Consider patient history and monitor for tolerability and side effects.
Start Mounjaro1:
- Initiate with the 2.5-mg dose
- After iv weeks on the ii.5-mg dose, increase to the 5-mg dose
If additional glycemic control is needed, you can proceed to increase the dose by 2.5-mg increments after at least 4 weeks on the electric current dose. The maximum dose is 15 mg in one case weekly.

Download a savings carte du jour for your eligible, commercially insured patients
SEE THE SAVINGS
Writing Mounjaro1

The amount to exist dispensed may be written as "4 pens" for a 28-day supply or "12 pens" for an 84-day supply.
Image depicting how to write the prescription for dissimilar doses of Mounjaro. All doses are injected subcutaneously once weekly and dispensed every bit i box or four pens for a 28-day supply. 2.5-mg dose NDC: 0002-1506-80; 5-mg dose NDC: 0002-1495-lxxx; vii.5-mg dose NDC: 0002-1484-80; ten-mg dose NDC: 0002-1471-fourscore; 12.v-mg dose NDC: 0002-1460-80; 15-mg dose NDC: 0002-1457-80.
More doses to assist achieve individual glycemic goals1
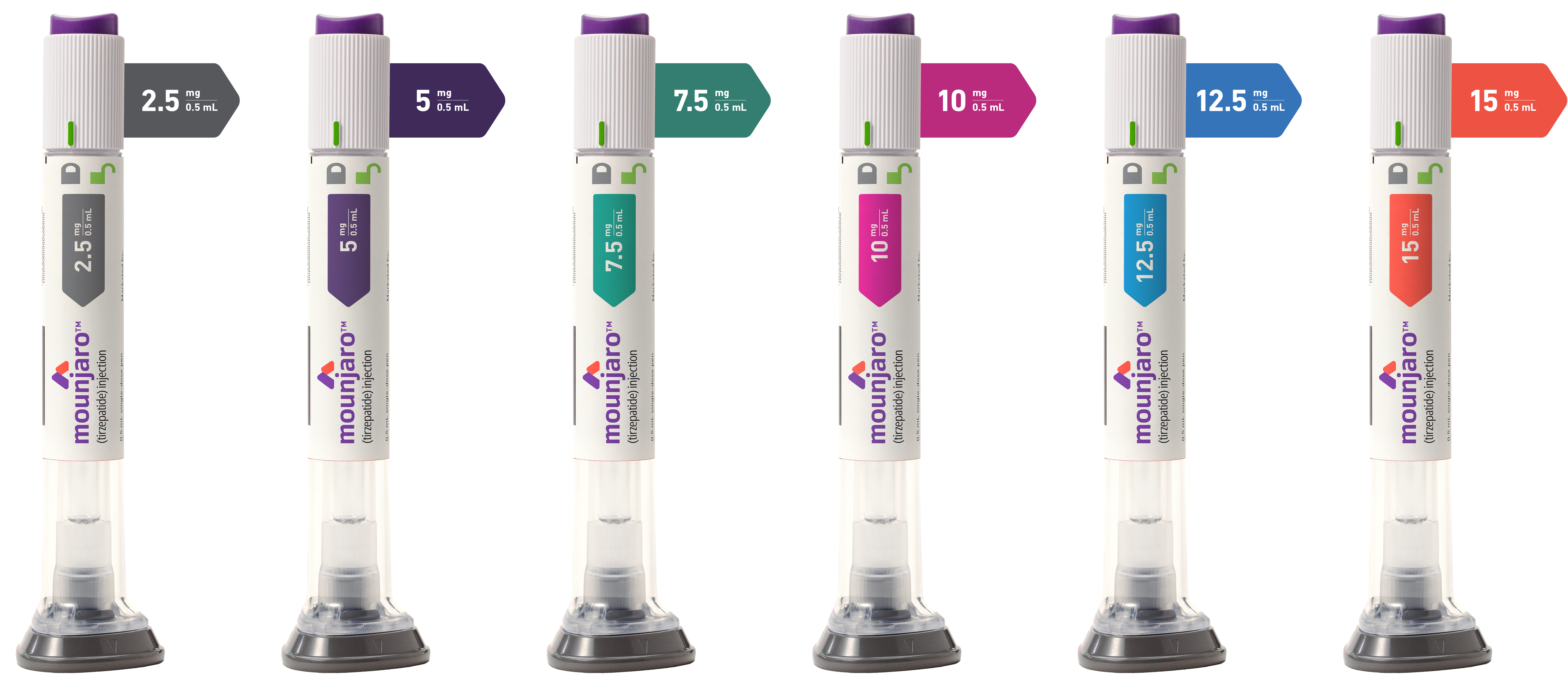
Image showing all 6 Mounjaro pen doses available: two.5 mg, 5 mg, seven.5 mg, 10 mg, 12.5 mg, and fifteen mg.
The 2.5 mg dose is for treatment initiation and is not intended for glycemic control.
- A unmarried-use, once-weekly, car-injection pen with a subconscious needle
- Available in 6 doses: a 2.5-mg starting dose and 5-mg, 7.5-mg, 10-mg, 12.v-mg, and xv-mg doses
1-Calendar month SUPPLY (28 DAYS)

Paradigm depicting one box of Mounjaro ii.v-mg pens.
NDC: 0002-1506-eighty
Number of days
i-calendar month supply (28 days)
Force
2.five mg/0.5 mL
Dosage form
Solution
SIG
Inject ii.five mg SC in one case weekly
Dispense quantity
ii mL for one month
Dispense quantity unit of mensurate
mL but
Number of boxes
1 box
3-Month SUPPLY (84 DAYS)

Image depicting three boxes of Mounjaro 2.5-mg pens.
5 mg NDC: 0002-1495-80
vii.v mg NDC: 0002-1484-80
10 mg NDC: 0002-1471-80
12.5 mg NDC: 0002-1460-80
15 mg NDC: 0002-1457-80
Number of days
- 1-month supply (28 days)
- 3-calendar month supply (84 days)
Strength
5 mg/0.five mL
vii.5 mg/0.v mL
10 mg/0.five mL
12.five mg/0.v mL
15 mg/0.5 mL
Dosage form
Solution
SIG
Inject v mg SC once weekly
Inject 7.5 mg SC once weekly
Inject 10 mg SC once weekly
Inject 12.5 mg SC in one case weekly
Inject 15 mg SC one time weekly
Dispense quantity
2 mL for ane month
6 mL for 3 months
Dispense quantity unit of measure
mL only
Number of boxes
1 box OR 3 boxes
Storing and dispensing the Mounjaro pen1
Store Mounjaro in a refrigerator at 36°F to 46°F (ii°C to 8°C)
- If needed, each single-dose pen tin can be stored unrefrigerated at temperatures not to exceed 86°F (30°C) for up to 21 days
- Do non freeze Mounjaro. Do not utilise Mounjaro if frozen
- Store Mounjaro in the original carton to protect from light
Getting patients startedone
As you get your patients started on Mounjaro, hither is some information that may help the conversation.

Information technology's taken once weekly1

It'south in a single-dose pen with a no-come across needletwo

It helps control blood sugarane
- Mounjaro can help lower A1C and blood glucose levels
- In clinical trials, boilerplate A1C reductions ranged from one.8% to 2.ane% for the v-mg dose, 1.7% to 2.4% for the x-mg dose, and 1.7% to 2.four% for the 15-mg dose
Select Important Rubber Information
Gamble of Thyroid C-cell Tumors: Counsel patients regarding the potential take chances for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (e.1000., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro. Such monitoring may increase the hazard of unnecessary procedures, due to the low test specificity for serum calcitonin and a high groundwork incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually take calcitonin values >50 ng/50. If serum calcitonin is measured and institute to be elevated, the patient should exist further evaluated. Patients with thyroid nodules noted on concrete examination or cervix imaging should also be further evaluated.
SC=subcutaneous; T2D=type 2 diabetes.
References:
- Mounjaro. Prescribing Data. Lilly USA, LLC.
- National Eye, Lung, and Blood Constitute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Report. National Institutes of Wellness; 1998. NIH publication 98-4083. Accessed February 25, 2022. http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf
- Van Gaal Fifty, Scheen A. Weight direction in blazon two diabetes:current and emerging approaches to handling. Diabetes Intendance. 2015;38(six):1161-1172.
- Wing RR, Await AHEAD Enquiry Group. Does lifestyle intervention improve health of adults with overweight/obesity and type 2 diabetes? Findings from the look Ahead randomized trial. Obesity. 2021;29(8):1246-1258.
- Mounjaro. Instructions for Employ. Lilly USA, LLC.
- Data on File. Lilly Usa LLC. DOF-TR-Usa-0005.
Indication
Mounjaro (tirzepatide), an injectable prescription medicine, is indicated as an adjunct to diet and do to better glycemic control in adults with type ii diabetes mellitus.
Limitations of Apply: Mounjaro has not been studied in patients with a history of pancreatitis. Mounjaro is not indicated for use in patients with blazon ane diabetes mellitus.
Important Prophylactic Data
WARNING: Hazard OF THYROID C-CELL TUMORS
In both male and female rats, tirzepatide causes dose-dependent and treatment-elapsing-dependent thyroid C-jail cell tumors at clinically relevant exposures. It is unknown whether Mounjaro causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has non been determined.
Mounjaro is contraindicated in patients with a personal or family unit history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type two (MEN ii). Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro.
Mounjaro is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with known serious hypersensitivity to tirzepatide or any of the excipients in Mounjaro.
Risk of Thyroid C-cell Tumors
Counsel patients regarding the potential risk for MTC with the employ of Mounjaro and inform them of symptoms of thyroid tumors (e.chiliad., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro. Such monitoring may increment the risk of unnecessary procedures, due to the depression test specificity for serum calcitonin and a loftier background incidence of thyroid illness. Significantly elevated serum calcitonin values may betoken MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical exam or neck imaging should also be farther evaluated.
Pancreatitis
Acute pancreatitis, including fatal and not-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-ane receptor agonists. Pancreatitis has been reported in Mounjaro clinical trials. Mounjaro has not been studied in patients with a prior history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher adventure for development of pancreatitis on Mounjaro. Find patients for signs and symptoms, including persistent severe abdominal hurting sometimes radiating to the back, which may or may not be accompanied past airsickness. If pancreatitis is suspected, discontinue Mounjaro and initiate appropriate management.
Hypoglycemia with Concomitant Employ of Insulin Secretagogues or Insulin
Concomitant use with an insulin secretagogue (e.thousand., sulfonylurea) or insulin may increment the risk of hypoglycemia, including astringent hypoglycemia. The take chances of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the adventure of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Hypersensitivity Reactions
Hypersensitivity reactions, sometimes severe, have been reported with Mounjaro in clinical trials. If hypersensitivity reactions occur, discontinue use of Mounjaro; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not employ in patients with a previous serious hypersensitivity to Mounjaro. Use caution in patients with a history of angioedema or anaphylaxis with a GLP-i receptor agonist considering information technology is unknown if such patients volition exist predisposed to these reactions with Mounjaro.
Acute Kidney Injury
Mounjaro has been associated with gastrointestinal agin reactions, which include nausea, vomiting, and diarrhea. These events may lead to dehydration, which if severe could cause acute kidney injury. In patients treated with GLP-one receptor agonists, at that place take been postmarketing reports of acute kidney injury and worsening of chronic renal failure, sometimes requiring hemodialysis. Some of these events have been reported in patients without known underlying renal disease. A bulk of reported events occurred in patients who had experienced nausea, airsickness, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of Mounjaro in patients with renal impairment reporting severe agin gastrointestinal reactions.
Severe Gastrointestinal Illness
Use of Mounjaro has been associated with gastrointestinal adverse reactions, sometimes severe. Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Mounjaro has non been studied in patients with non-proliferative diabetic retinopathy requiring astute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Patients with a history of diabetic retinopathy should exist monitored for progression of diabetic retinopathy.
Acute Gallbladder Disease
In clinical trials, acute gallbladder disease was reported by 0.6% of Mounjaro-treated patients and 0% of placebo-treated patients. If cholelithiasis is suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated.
The virtually common adverse reactions reported in ≥v% of Mounjaro-treated patients in placebo-controlled trials were nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and intestinal hurting.
Drug Interactions
When initiating Mounjaro, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia. Mounjaro delays gastric emptying, and thereby has the potential to impact the assimilation of concomitantly administered oral medications, so caution should be exercised.
Pregnancy
Limited data on Mounjaro employ in pregnant women are available to inform on drug-associated risk for major nativity defects, miscarriage, or other adverse maternal or fetal outcomes. Based on brute reproduction studies, in that location may be risks to the fetus from exposure to tirzepatide. Use only if potential benefit justifies the potential risk to the fetus.
Lactation
There are no data on the presence of tirzepatide in homo milk, the effects on the breastfed infant, or the furnishings on milk product. The developmental and health benefits of breastfeeding should be considered forth with the female parent'south clinical need for Mounjaro and any potential adverse effects on the breastfed infant from Mounjaro or from the underlying maternal condition.
Females of Reproductive Potential
Advise females using oral hormonal contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception for 4 weeks subsequently initiation and for 4 weeks subsequently each dose escalation.
Pediatric Use
Safety and effectiveness of Mounjaro have non been established and use is not recommended in patients less than 18 years of age.
Source: https://www.mounjaro.com/hcp/getting-patients-started

0 Response to "How To Get A Prescription For Mounjaro"
Post a Comment